|
◎ 文/陳柏瑋
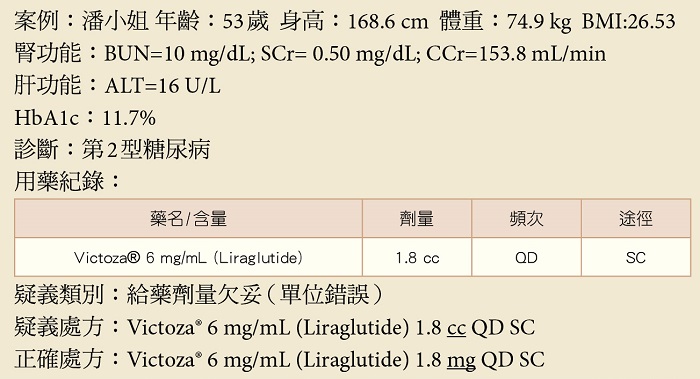
3.疑義說明:
⑴此病人因第2型糖尿病開立Victoza® (胰妥善),劑量1.8 cc 每日一次皮下注射。
⑵胰妥善每cc包含6毫克的liraglutide,1.8cc為10.8毫克,故應將處方修改為1.8毫克每日一次皮下注射。
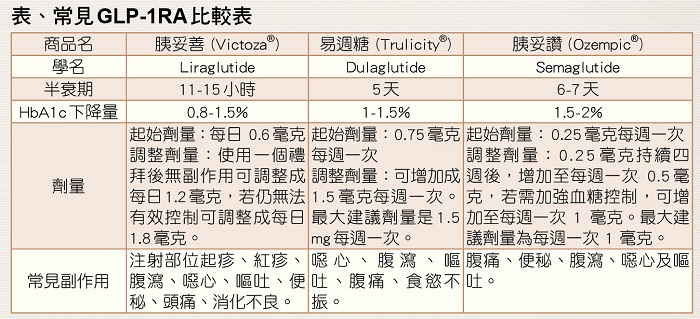
檢驗項目參考值:
BUN (blood urea nitrogen): 7-20 mg/dL; SCr (serum creatinine): male:
0.7-1.5 mg/dL; female: 0.5-1.2 mg/dL; CCr (Creatinine clearance
rate): >60 mL/min; ALT (alanine aminotransferase): 0-40 U/L; AST (aspartate
aminotransferase): 5-45 U/L;K: 3.5-5.0 mEq/L
參考資料:
1. Victoza [dosing and titration guide]. North Carolina, USA: Novo
Nordisk; 2017.
2. Trulicity [dosing and titration guide]. Indiana, USA: Eli Lilly
and Company; 2020.
3. Ozempic [dosing and titration guide]. Bagsvaerd, Denmark: Novo
Nordisk; 2020.
4. Kathleen Dungan (2023). Glucagon-like peptide 1-based therapies
for the treatment of type 2 diabetes mellitus. J.P. Forman(Ed.),
Uptodate. Retrieved April 26, 2023, from https://www.uptodate.com/contents/glucagon-like-peptide-1-based-therapies-for-the-treatment-of-type-2-diabetes-mellitus/
(全文完)
(本文作者為臺北榮民總醫院藥學部臨床藥師 / 陽明大學藥理所碩士)
回首頁 |